The beauty and challenge of complexity
The dream of creating an “organ in a dish” dates back to 1907, when Henry Van Peters Wilson first demonstrated that dissociated sponge cells could reorganize into an entire organism. But over 100 years passed before researchers successfully modeled intestinal tissue using adult stem cells and the field of organoid development was off to the races. For the first time, these tiny 3D structures were able to recreate the functional structures of an organ, essentially becoming mini-guts, mini-brains or mini-livers.
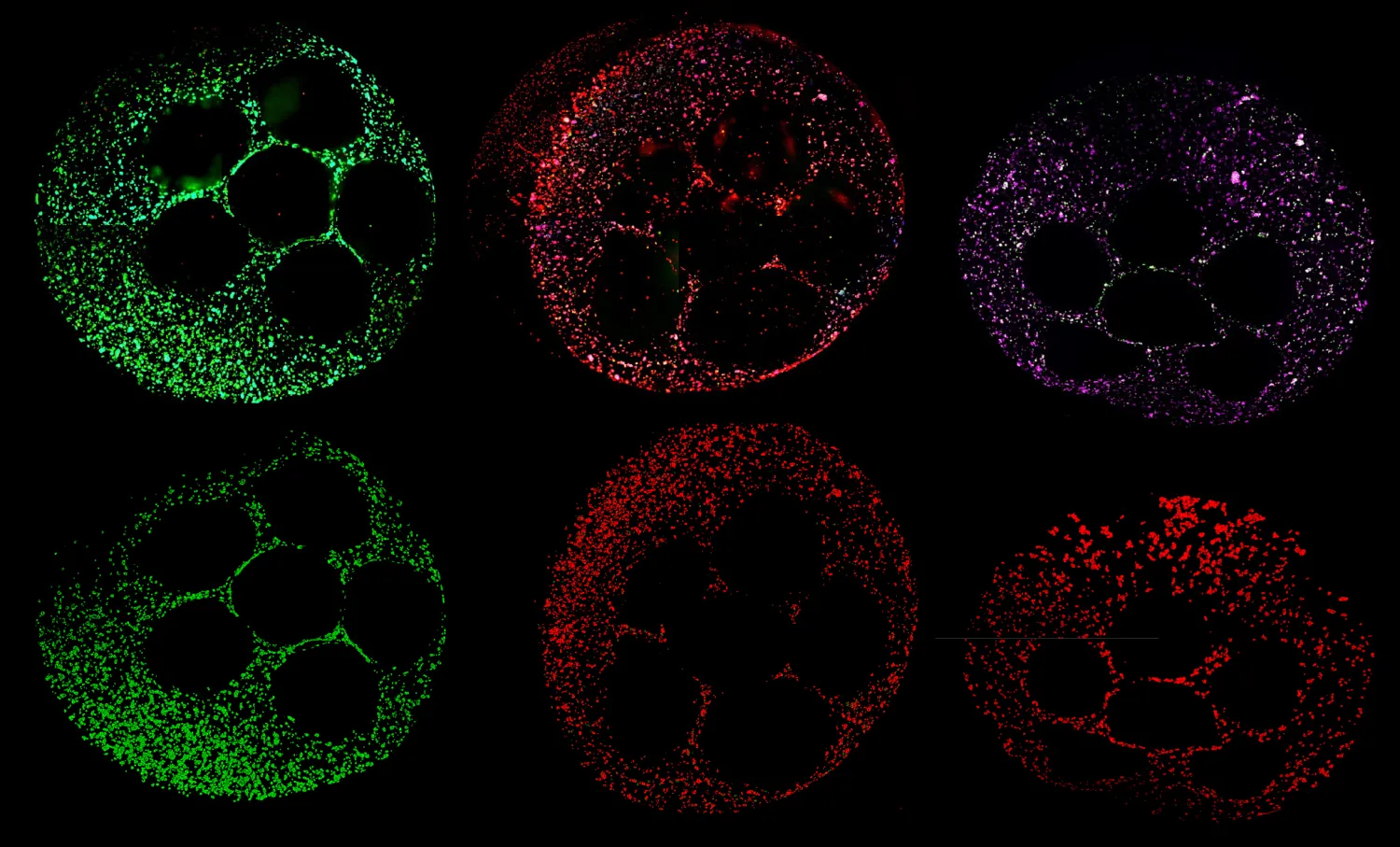
Today, organoid research has become a key method for studying the underlying processes of disease—and potentially intervening. Organoids are used to model diseases like cystic fibrosis, cancer and Alzheimer's, to create customized cell therapies and, of course, to screen for drug toxicity. Unlike other model systems, organoids can mimic the complexity of a full human biological system, captured in the mesmerizing images that convey the wonder of that complexity.
Compared with conventional in vivo methods, liver organoids can predict DILI with extraordinary accuracy—in some cases, nearly 89%. But organoids have yet to take off as a widespread preclinical research tool. Creating the culture conditions to guide stem cells through all the correct developmental stages of becoming a usable organoid requires significant expertise that’s often only found in specialized labs. Additionally, most organoid cultures are still grown manually, which demands substantial labor and time. Artificial intelligence is playing a key role in expanding the insights organoids can reveal, but even AI can’t perform without a high volume of quality data to feed the models, meaning data generation and the protocols to guide it remain a bottleneck.